Another pandemic — one that could result in more deaths and severe illnesses than COVID-19 — looms. But unlike COVID-19, which took the world by surprise, this pandemic is already in plain sight.
That’s how the challenges of anti-microbial resistance was described in February of 2022 by the Boston Consulting Group and underlines why this is extremely important for the global society to find betters ways to combat and solve.
From the very inception of grit42 in 2014 we have been partners in big Innovative Medicines Initiatives (IMI) projects within Anti Microbial Resistance (AMR) and currently we’re partners in both the IMI AMR COMBINE as well as the IMI AMR ERA4TB projects with a combined list of around 40 partners across Big Pharma, Universities, Governmental Institutions and SMEs.
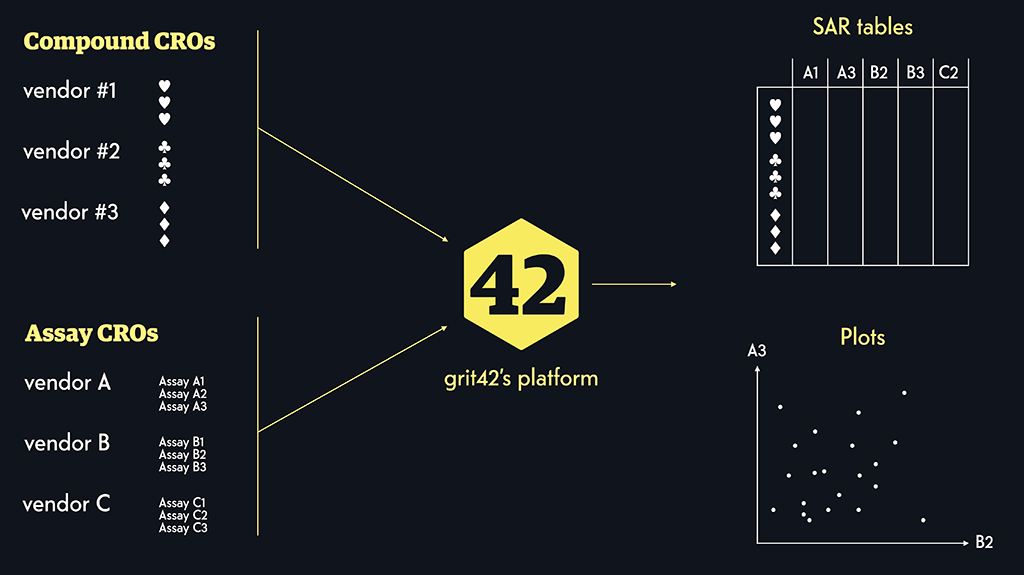
In both projects the grit42 platform play a central role in ensuring the storage of both pre-clinical and clinical data in a structured and FAIR format. As part of the work under the IMI AMR Accelerator projects we have – together with other data management partners like Fraunhofer and C-Path – extended the grit42 platform and added new valuable features – both for the IMI projects but also for grit42 and our current and future customers.
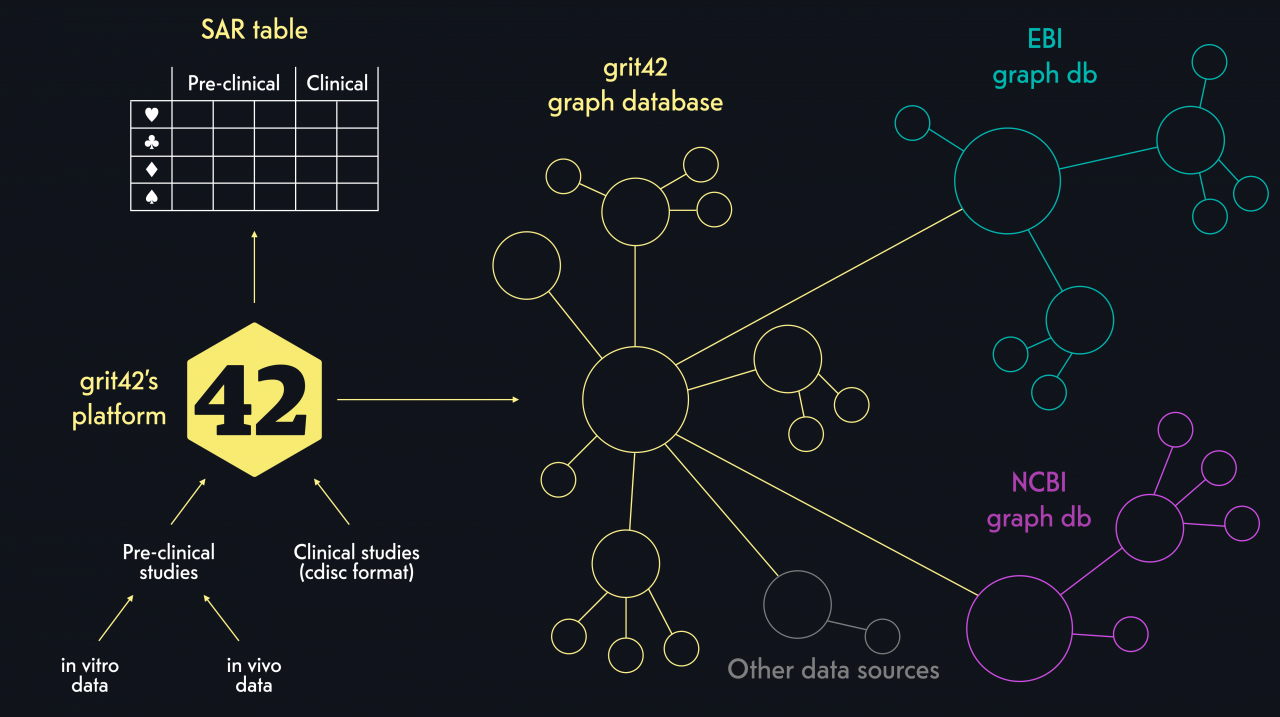
Together with our partners from Fraunhofer, we created a knowledge graph where the pre-clinical and clinical research data in the grit42 platform has been turned into a graph and linked with other relevant and related data from other data sources to create a resource for very broad data analysis.
The AMR Data Management group has already developed a set of database and visualisation tools based on the Knowledge Graph standards.
The IMI AMR projects runs until 2025, so we expect plenty of new and great features to be added to the platform from the close collaborations across all the IMI partners.




Due to the growing issues with microbial resistance where some drugs simply lose effect, it is common to use combination therapy (mixing drugs) in the AMR field. However, this poses a bit of a challenge in the classic biopharma data systems as all “compounds” being tested need to be registered. And in this case it’s not new compounds but a mixture of other compounds already in the system. Together with C-Path we have extended the grit42 platform in a way where we can now – not only – handle combinations of compounds but also combination of the batch and the actual vials used to create a new “combination batch/vial”.
In the IMI ERA4TB project data from both pre-clinical and clinical research are collected and need to be stored in the grit42 platform in order to enable cross study analysis in both areas. But equally important this ensures the ability to perform data analysis across pre-clinical and clinical data and hence translation from animal studies to the human clinical trials.